
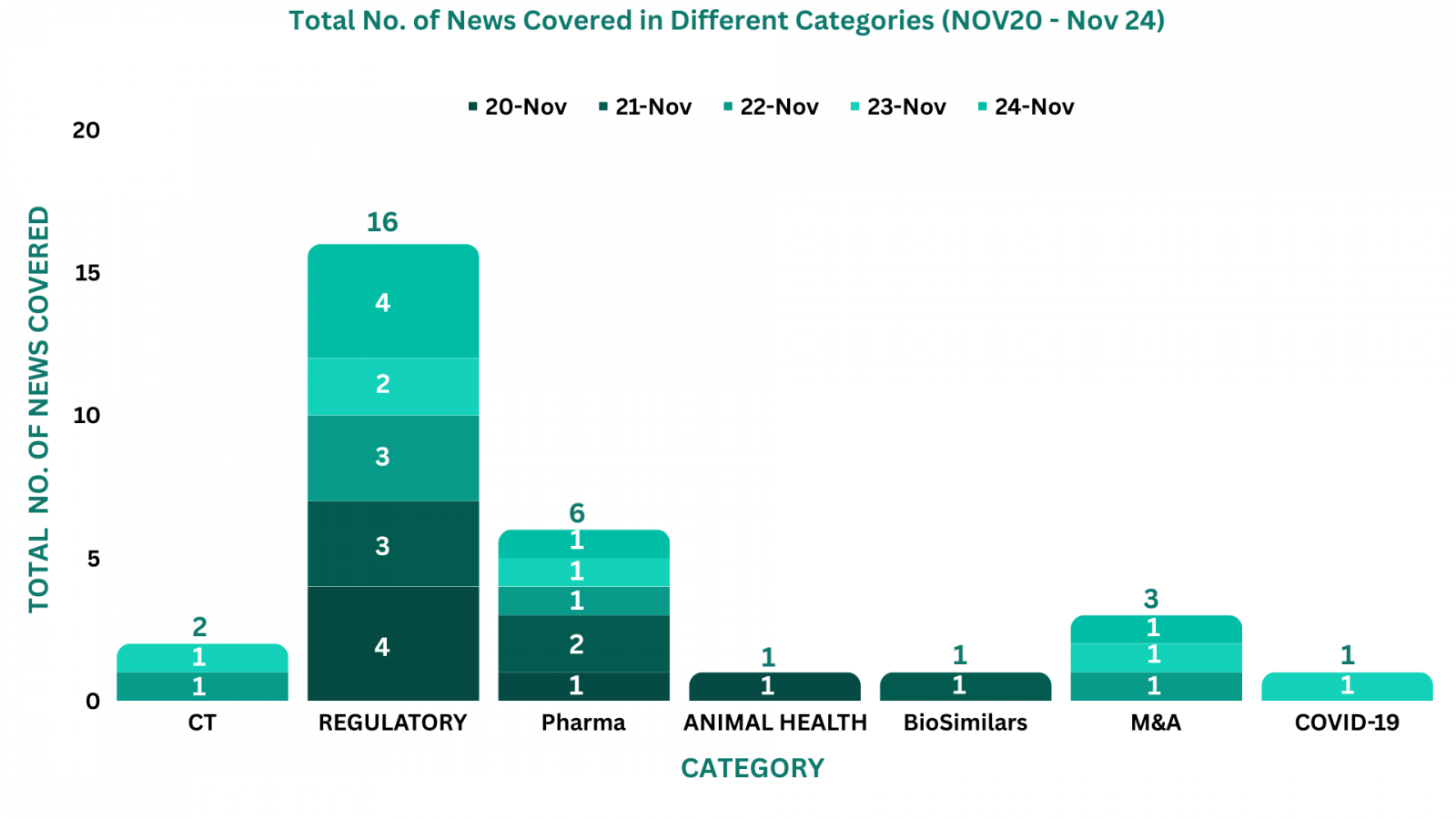
PharmaShots Weekly Snapshots (November 20 – November 24, 2023)
This week PharmaShots’ news was all about the updates on regulatory, Animal Health, Pharma, BioSimilars, M&A, Clinical Trials and COVID-19. Check out our full report below:

The European Commission Approved BeiGene’s Brukinsa with Obinutuzumab for the Treatment of Follicular Lymphoma
Read More: BeiGene
AstraZeneca’s Truqap (capivasertib) + Fulvestrant Received the US FDA's Approval for the treatment of advanced HR-positive breast cancer
Read More: AstraZeneca
Masimo Received the US FDA's Approval for OTC and Prescription use for their W1 Medical Watch
Read More: Masimo
Almirall’s Ebglyss (lebrikizumab) Received EC’s Approval for the Treatment of Moderate-to-severe Atopic Dermatitis
Read More: Almirall
The European Commission Approved Ascendis’ Yorvipath for the Treatment of Chronic Hypoparathyroidism
Read More: Ascendis
The US FDA Oncologic Drugs Advisory Committee (ODAC) will review sBLA for Abecma jointly developed by BMS & 2seventy Bio
Read More: BMS & 2seventy Bio
Pixyl's Pixyl.Neuro AI-powered Brain MRI Software Received the US FD’s 510(k) Approval
Read More: Pixyl
European Patent Office Cancelled Moderna mRNA Patent
Read More: Moderna
The US FDA Approved GE Healthcare’s Digital Expert Access
Read More: GE Healthcare
The US FDA Approved Senhwa Bioscience' IND for P-II Study of Silmitasertib in Patients with Community-Acquired Pneumonia (CAP) Caused by Viral Infection
Read More: Senhwa Bioscience
The European Commission (EC) has Approved the Market Authorization for Trastuzumab, a Biosimilar Developed by EirGenix
Read More: EirGenix
The EC Approved Boehringer Ingelheim’s Senvelgo for the treatment of Feline diabetes
Read More: Boehringer Ingelheim
Siemens’s Biograph Vision.X PET/CT Scanner Receives the US FDA Approval
Read More: Siemens
The NMPA Accepts Innovent's NDA for IBI351 & Grants Priority Review Designation
Read More: Innovent
The NMPA Approves Everest Medicine’s Nefecon for the Treatment of Primary IgA Nephropathy
Read More: Everest Medicine
The EC Approves Vertex’s Kaftrio + Ivacaftor for the Treatment of Cystic Fibrosis (CF)
Read More: Vertex

To develop CRE-DR-B, EpitoMAP, Animal Allergy Clinical Laboratories (AACL), and Kogyo entered into a collaboration and license agreement
Read More: Animal Allergy Clinical Laboratories & Kogyo

MediciNova Reports New Data from the P-II Study of MN-166 (ibudilast) for glioblastoma at Annual Meeting Of SNO
Read More: MediciNova
New data from P-III study (INDIGO) presented by Servier to treat Glioma Patients
Read More: Servier
MIRA Pharmaceutical Entered into An Exclusive Licensing Agreement with MIRALOGX for Ketamir-2
Read More: MIRA Pharmaceutical & MIRALOGX
Beigene to Purchase the Exclusive Worldwide License for Ensem Therapeutics' Investigational Oral CDK2 Inhibitor EXT-197
Read More: Beigene & Ensem Therapeutics
PharmAbcine Published the results of Preclinical Data of PMC-403 for Clarkson Disease
Read More: PharmAbcine
Simcere Collaborates with Connect Biopharm & Enters into an Exclusive License Agreement for Rademikibart
Read More: Simcere & Connect Biopharm

In Europe, Sandoz Launches a High-Concentration Formulation of Hyrimoz
Read More: Sandoz

Merck Announces Acquisition of Caraway Therapeutics
Read More: Merck
Freeline to be Acquired by Syncona in All Cash Transactions
Read More: Freeline
The Ownership of GSK4381406 is Reclaimed by Sosei Heptares from GSK
Read More: GSK & Sosei Heptares
Innovent Reports the Result P-II Study of Mazdutide (IBI362) On Chinese Patients with Type-2 Diabetes (T2D)
Read More: Innovent
T3 Pharma Aquired by Boehringer Ingelheim for the Amount of $508.2M
Read More: Boehringer Ingelheim & T3 Pharma

Junshi Biosciences Publishes the Result of VV116 in P-III to treat COVID-19 in The Lancet
Read More: Junshi Biosciences
Related Post:- PharmaShots Weekly Snapshots (November 14 – November 17, 2023)
Tags

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.













